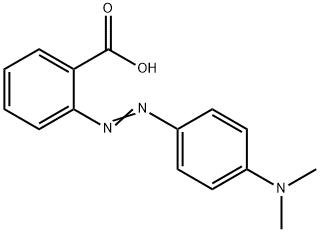
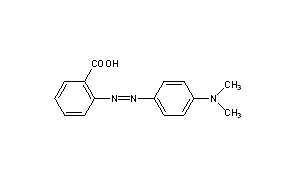
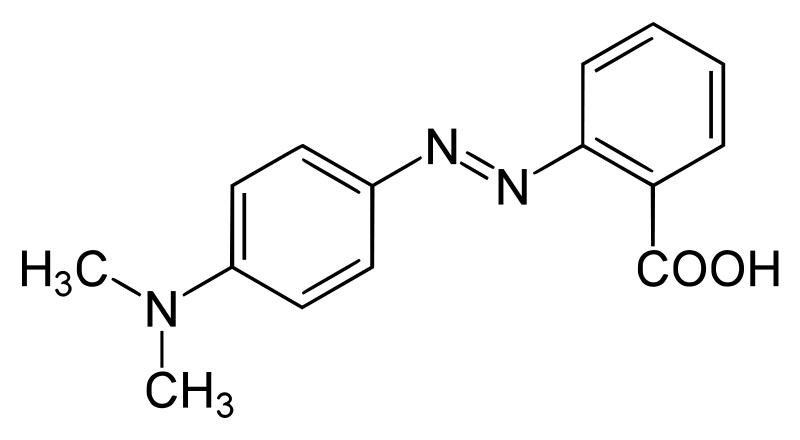
SOLVED:Methyl red has a pKa of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0 -mL
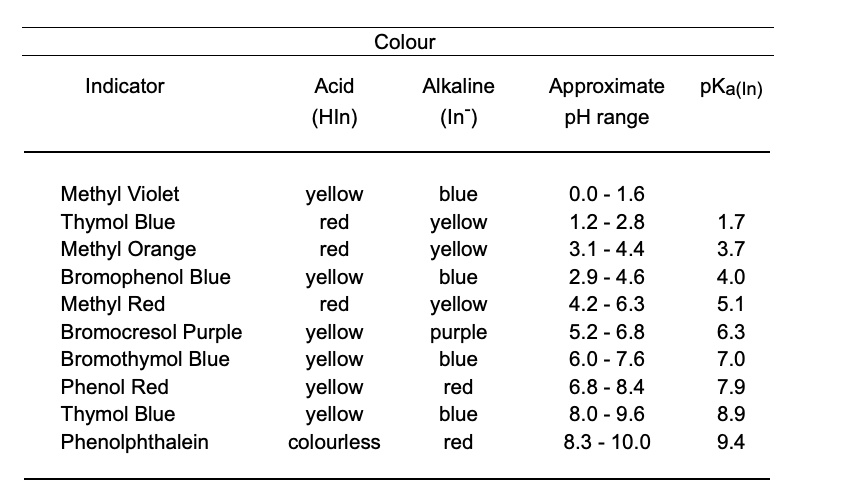
SOLVED: Colour Indicator Acid (HIn) Alkaline (In" ) Approximate pH range pKa(ln) Methyl Violet Thymol Blue Methyl Orange Bromophenol Blue Methyl Red Bromocresol Purple Bromothymol Blue Phenol Red Thymol Blue Phenolphthalein yellow

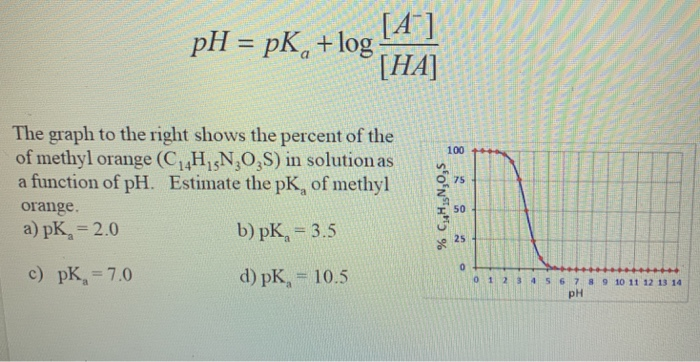
UV-Vis pKa of methyl red 1 .pdf - UV-vis Spectroscopy: pKa of Methyl Red UV-vis spectroscopy Etotal = Eelectronic Evibrational Erotational The | Course Hero
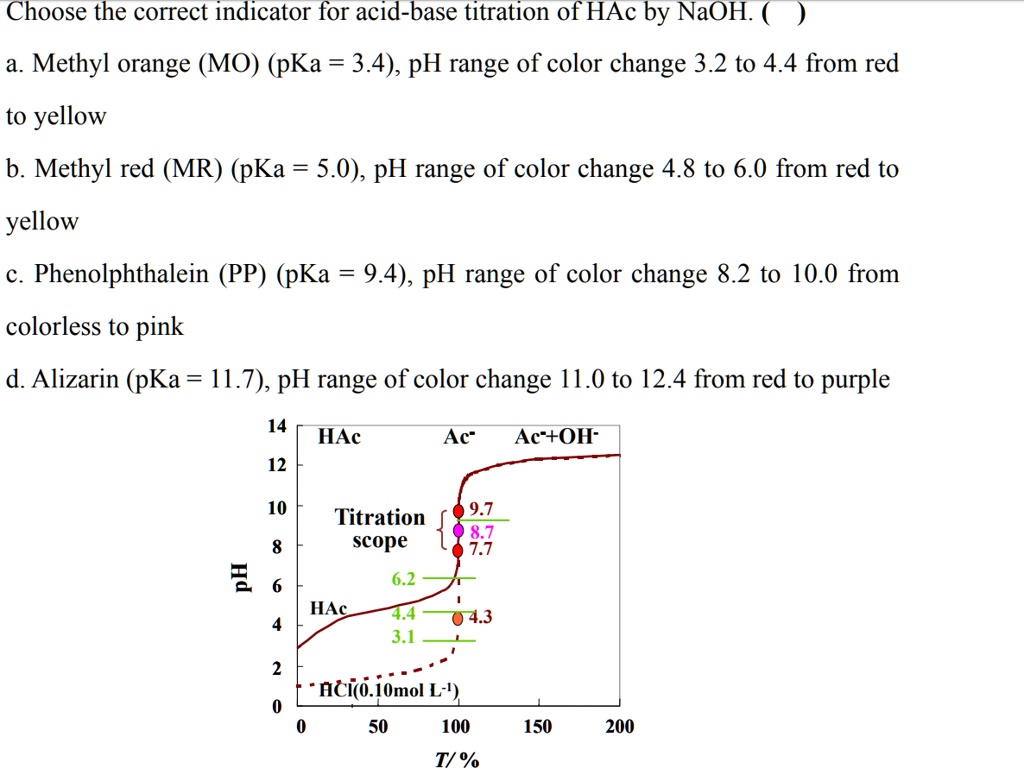
SOLVED: Choose the correct indicator for acid-base titration of HAc by NaOH: Methyl orange (MO) (pKa = 3.4), pH range of color change 3.2 to 4.4 from red to yellow b. Methyl

Methyl red has the following structure: It undergoes a color change from red to yellow as a solution gets more basic. Calculate an approximate pH range for which methyl red is useful.

VOLUMETRIC ANALYSIS Volumetric analysis is an analysis in which the amount of the unknown is calculated from a known volume of added solution. - ppt video online download

Structures of diazo pH indicators. (A) Main structure of methyl orange... | Download Scientific Diagram
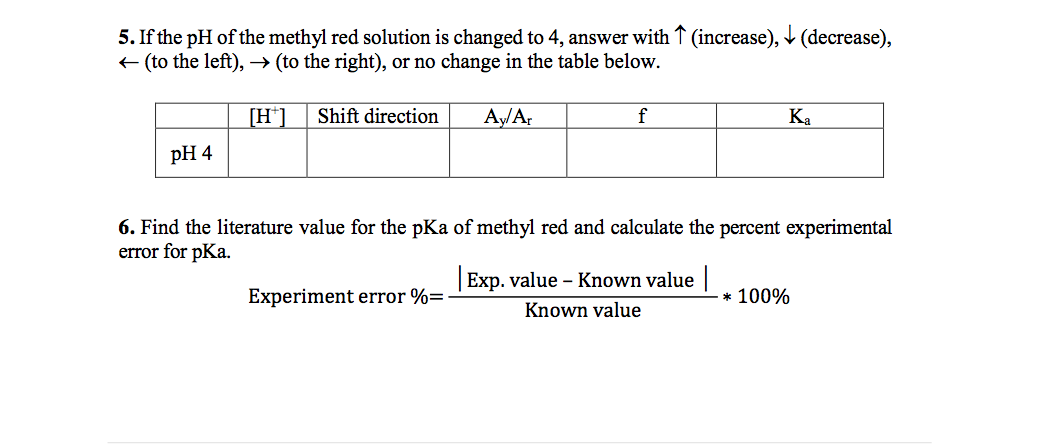
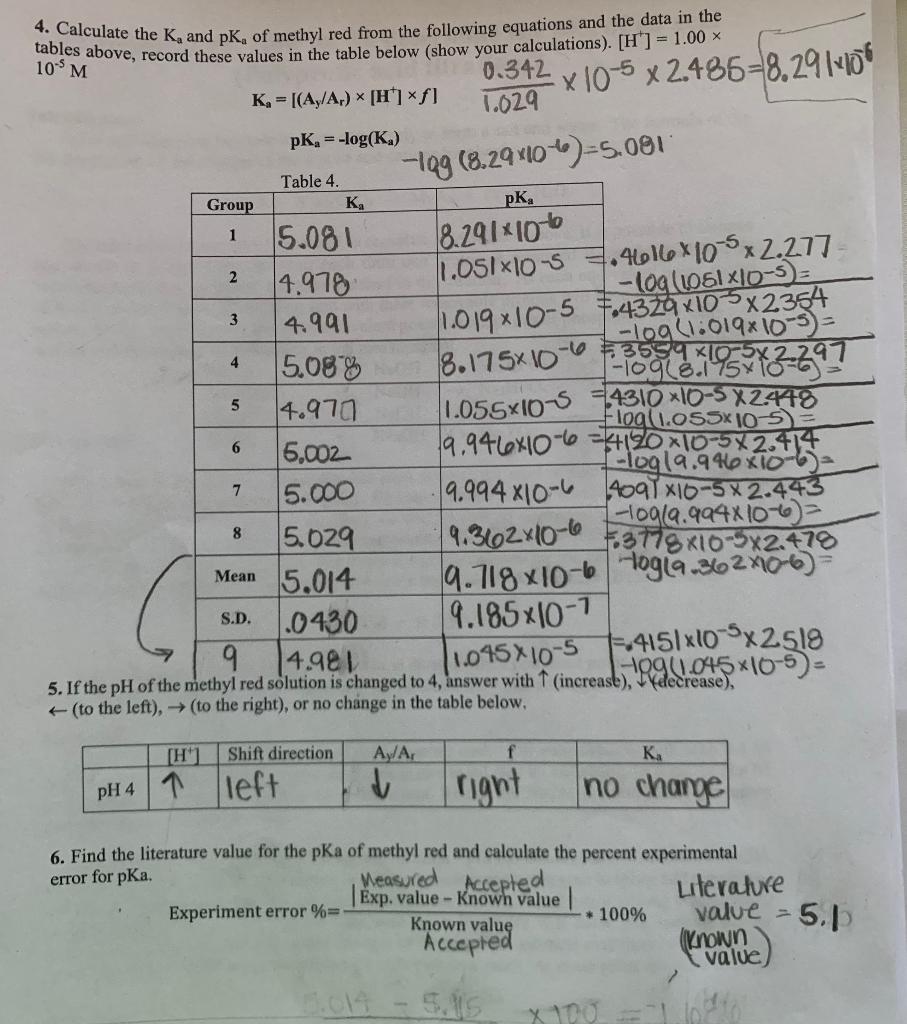
![SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] = SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] =](https://cdn.numerade.com/ask_images/a0a70d476fd049fea11245c72f687323.jpg)
SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] =
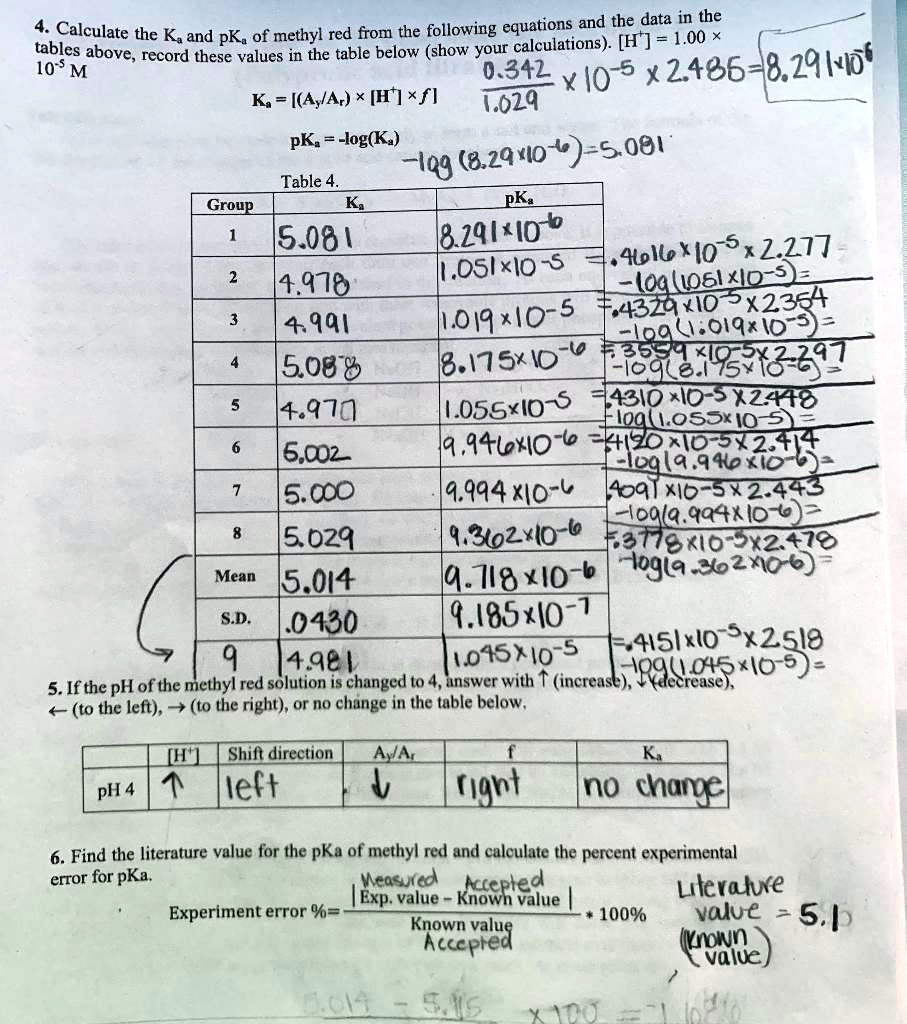
SOLVED: and the data j,8l in the Calculate the K and pK, of methyl red fom the following equationsi tables above, record Tesekvalues €t the cable below (show your calculations) [H7 =

Table 3 from Natural Plant Extracts as Acid-Base Indicator and Determination of Their pKa Value | Semantic Scholar